Home News & Views Engaging patients in health research activity
Engaging patients in health research activity

Paula Rogers, Research Study Nurse, James Lind Care
I was very fortunate to receive funding from FoNS in 2013, as part of the Patient’s First Programme. Since then (and the wonderful support received from Jo Odell), I have been a keen follower of FoNS.
I retired from the NHS at the end of 2020 and wanted to share my experience of a new way of working in health research.
The challenges and changes of the pandemic
The COVID pandemic has been stressful for many patients, carers, and healthcare workers for a variety of reasons. The success of the COVID vaccination programme has pushed health research to greater prominence in the minds of many, with the general public reaching out to support COVID research initiatives.
In addition, social media platforms such as Facebook, Twitter and Instagram have become a ‘go-to’ place for patients to discover more about their health conditions. Such social media platforms have the ability to inform patients about potential research studies and my new role is related to this relatively new way of engaging patients in health research activities.
While being involved in research studies is not for everyone, many patients feel it is a way of being able to help the progress of science or give something back to health services.
Participation and engagement in clinical research
The British Research Panel is a voluntary British patient community with more than 15,000 members. Members of the public will see the British Research Panel promoted via social media platforms and if they would like to understand more about the organisation, they are invited to register their interest to become a member. The British Research Panel is supported by James Lind Care, a specialised clinical research patient organisation operating in Europe. Registered members receive regular newsletters and are invited to complete anonymous surveys to help James Lind Care understand the research needs of community-based patients.
Patients who are interested in research participation complete a health questionnaire; if their responses meet inclusion criteria for a specific trial, potential participants will be contacted by a research nurse. The British Research Panel adheres to data protection regulations and patients provide written consent to allow their health information to be stored and shared with research sites. Online patient research communities, with a specific aim of informing members of research opportunities, provide a safe environment for potential participants to digest information before deciding whether to progress their interest.
Dedicated nurses guiding patients in their clinical trial engagement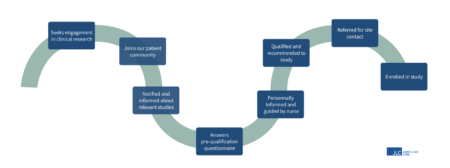
I work with a dedicated team of research nurses throughout Europe, and our role is to contact patients who have expressed an interest in research participation via the patient community platforms. We speak with many patients each day and provide support and information related to clinical trials. Some patients need signposting towards seeking help for management of their health condition, other patients want to tell their story of their health experiences, the majority of the patients are seeking information about health research to help determine if research participation is for them. Patients feel empowered to make their own decisions regarding research involvement following their self-referral and it is humbling for the research nurses to be part of this journey. The research nurses have contact with the research sites and sponsors of the research studies, but it is the unique relationship with patients that makes this role so special. The figure below depicts a typical patient and research nurse journey through the British Research Panel.
Please feel free to contact me if you would like further information:[email protected]
Comments are closed.

